Lead
|
||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Metallic gray |
||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | lead, Pb, 82 | |||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˈlɛd/ led | |||||||||||||||||||||||||||||||||||||||||||||
| Element category | post-transition metal | |||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 14, 6, p | |||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 207.2g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p2 | |||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 4 (Image) | |||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 11.34 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 10.66 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 600.61 K, 327.46 °C, 621.43 °F | |||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2022 K, 1749 °C, 3180 °F | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 4.77 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 179.5 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 26.650 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4, 2 (Amphoteric oxide) | |||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.33 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 715.6 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1450.5 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3081.5 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 175 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 146±5 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 202 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic | |||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 208 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 35.3 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 28.9 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 16 GPa | |||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 5.6 GPa | |||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 46 GPa | |||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.44 | |||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.5 | |||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 38.3 MPa | |||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7439-92-1 | |||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of lead | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||

Lead is a main-group element with symbol Pb (Latin: plumbum) and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air. Lead has a shiny chrome-silver luster when it is melted into a liquid.
Lead is used in building construction, lead-acid batteries, bullets and shots, weights, as part of solders, pewters, fusible alloys and as a radiation shield. Lead has the highest atomic number of all of the stable elements, although the next higher element, bismuth, has a half-life that is so long (much longer than the age of the universe) that it can be considered stable. Its four stable isotopes have 82 protons; a "magic number" in the nuclear shell model of atomic nuclei.
Lead is a poisonous substance to animals. It damages the nervous system and causes brain disorders. Excessive lead also causes blood disorders in mammals. Like the element mercury, another heavy metal, lead is a potent neurotoxin that accumulates both in soft tissues and the bones. Lead poisoning has been documented from ancient Rome, ancient Greece, and ancient China.
Contents |
Characteristics
Lead is bright and silvery when freshly cut but the surface rapidly tarnishes in air to produce the commonly observed dull luster normally associated with lead. It is a dense, ductile, very soft, highly malleable, bluish-white metal that has poor electrical conductivity when compared to most other metals. This metal is highly resistant to corrosion, and because of this property, it is used to contain corrosive liquids (for example, sulfuric acid). Because lead is very malleable and resistant to corrosion it is extensively used in building construction – for example in the external coverings of roofing joints.
Metallic lead can be toughened by addition of small amounts of antimony, or of a small number of other metals such as calcium. All isotopes of lead, except for lead-204, can be found in the end products of the radioactive decay of the even heavier elements, uranium and thorium.
Creation
Lead-204 is believed to have been created in supernovae by the r-process of nucleosynthesis. Lead-206 was also created in stars by the s-process of nucleosynthesis.
Isotopes
Lead can be found or produced in many isotopes, with four of them being stable. The four stable isotopes of lead are 204Pb, 206Pb, 207Pb, and 208Pb with 204Pb regarded as completely primordial lead, and 206, 207, 208 being formed probably from the radioactive decay of two isotopes of uranium (U-235 and U-238) and one isotope of thorium (Th 232).
The one common radiogenic isotope of lead, 202Pb, has a half life of about 53,000 years.[1]
Chemistry
Various oxidized forms of lead are easily reduced to the metal. An example is heating PbO with mild organic reducing agents such as glucose. A mixture of the oxide and the sulfide heated together will also form the metal.[2]
- 2 PbO + PbS → 3 Pb + SO2
Metallic lead is attacked (oxidized) only superficially by air, forming a thin layer of lead oxide that protects it from further oxidation. The metal is not attacked by sulfuric or hydrochloric acids. It does, however, dissolve in nitric acid with the evolution of nitric oxide gas to form dissolved Pb(NO3)2.
- 3 Pb + 8 H+ + 8 NO−3 → 3 Pb2+ + 6 NO−3 + 2 NO + 4 H2O
When heated with nitrates of alkali metals, metallic lead oxidizes to form PbO (also known as litharge), leaving the corresponding alkali nitrite. PbO is representative of lead's +2 oxidation state. It is soluble in nitric and acetic acids, from which solutions it is possible to precipitate halide, sulfate, chromate, carbonate (PbCO3), and basic carbonate (Pb3(OH)2(CO3)2) salts of lead. The sulfide can also be precipitated from acetate solutions. These salts are all poorly soluble in water. Among the halides, the iodide is less soluble than the bromide, which, in turn, is less soluble than the chloride.[3]
Lead(II) oxide is also soluble in alkali hydroxide solutions to form the corresponding plumbite salt.[2]
- PbO + 2 OH− + H2O → Pb(OH)2−4
Chlorination of plumbite solutions causes the formation of lead's +4 oxidation state.
- Pb(OH)2−4 + Cl2 → PbO2 + 2 Cl− + 2 H2O
Lead dioxide is representative of the +4 oxidation state, and is a powerful oxidizing agent. The chloride of this oxidation state is formed only with difficulty and decomposes readily into lead(II) chloride and chlorine gas. The bromide and iodide of lead(IV) are not known to exist.[3] Lead dioxide dissolves in alkali hydroxide solutions to form the corresponding plumbates.[2]
- PbO2 + 2 OH− + 2 H2O → Pb(OH)2−6
Lead also has an oxide with mixed +2 and +4 oxidation states, red lead (Pb3O4), also known as minium.
Lead readily forms an equimolar alloy with sodium metal that reacts with alkyl halides to form organometallic compounds of lead such as tetraethyl lead.[4]
Chloride complexes
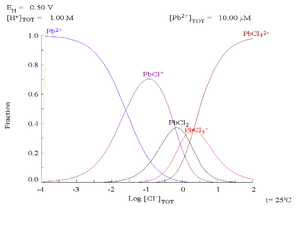
Lead(II) forms a series of complexes with chloride, the formation of which alters the corrosion chemistry of the lead. This will tend to limit the solubility of lead in saline media.
| Pb2+ + Cl− → PbCl+ | K1 = 12.59 |
| PbCl+ + Cl− → PbCl2 | K2 = 14.45 |
| PbCl2 + Cl− → PbCl3− | K3 = 3.98 ×10−1 |
| PbCl3− + Cl− → PbCl42− | K4 = 8.92 × 10−2 |
Phase diagrams of solubilities
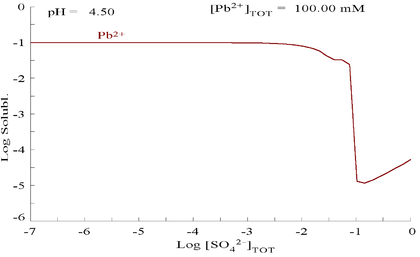
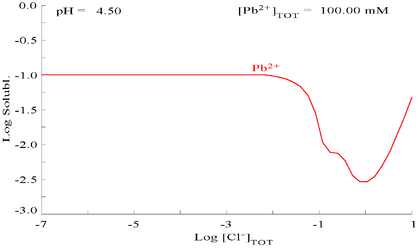
Lead(II) sulfate is poorly soluble, as can be seen in the following diagram showing addition of SO42− to a solution containing 0.1 M of Pb2+. The pH of the solution is 4.5, as above that, Pb2+ concentration can never reach 0.1 M due to the formation of Pb(OH)2. Observe that Pb2+ solubility drops 10,000 fold as SO42− reaches 0.1 M.
 |
 |
| Plot showing aqueous concentration of dissolved Pb2+ as a function of SO42− [5] | Diagram for lead in sulfate media[5] |
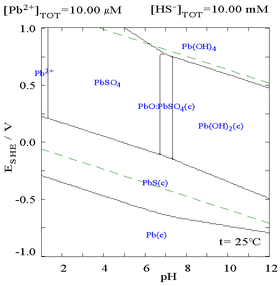
Here it can be seen that the addition of chloride can lower the solubility of lead, however in chloride rich media (such as aqua regia) the lead can become soluble again as anionic chlorocomplexes.
 |
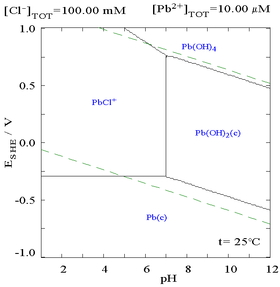 |
| Diagram showing the solubility of lead in chloride media. The lead concentrations are plotted as a function of the total chloride present.[5] | Pourbaix diagram for lead in chloride (0.1 M) media[5] |
History
Lead has been commonly used for thousands of years because it is widespread, easy to extract and easy to work with. It is highly malleable and ductile as well as easy to smelt. Metallic lead beads dating back to 6400 BC have been found in Çatalhöyük in modern-day Turkey.[7] In the early Bronze Age, lead was used with antimony and arsenic. Lead is mentioned in the Book of Exodus (15:10).
The largest preindustrial producer of lead was the Roman economy, with an estimated output per annum of 80,000 t, which was typically won as a by-product of silver smelting.[8][9][10] Roman mining activities occurred in Central Europe, Roman Britain, the Balkans, Greece, Asia Minor; Hispania alone accounted for 40% of world production.[8]
Roman lead pipes often bore the insignia of Roman emperors (see Roman lead pipe inscriptions). Lead plumbing in the Latin West may have been continued beyond the age of Theoderic the Great into the medieval period.[11] Many Roman "pigs" (ingots) of lead figure in Derbyshire lead mining history and in the history of the industry in other English centers. The Romans also used lead in molten form to secure iron pins that held together large limestone blocks in certain monumental buildings. In alchemy, lead was thought to be the oldest metal and was associated with the planet Saturn.
Lead's symbol Pb is an abbreviation of its Latin name plumbum for soft metals; originally it was plumbum nigrum (literally, "black plumbum"), where plumbum candidum (literally, "bright plumbum") was tin. The English words "plumbing", "plumber", "plumb", and "plumb-bob" also derive from this Latin root.
Occurrence

Metallic lead does occur in nature, but it is rare. Lead is usually found in ore with zinc, silver and (most abundantly) copper, and is extracted together with these metals. The main lead mineral is galena (PbS), which contains 86.6% lead. Other common varieties are cerussite (PbCO3) and anglesite (PbSO4).
Ore processing

Most ores contain less than 10% lead, and ores containing as little as 3% lead can be economically exploited. Ores are crushed and concentrated by froth flotation typically to 70% or more. Sulfide ores are roasted, producing primarily lead oxide and a mixture of sulfates and silicates of lead and other metals contained in the ore.[12]
Lead oxide from the roasting process is reduced in a coke-fired blast furnace.[13] This converts most of the lead to its metallic form. Three additional layers separate in the process and float to the top of the metallic lead. These are slag (silicates containing 1.5% lead), matte (sulfides containing 15% lead), and speiss (arsenides of iron and copper). These wastes contain concentrations of copper, zinc, cadmium, and bismuth that can be recovered economically, as can their content of unreduced lead.[12]
Metallic lead that results from the roasting and blast furnace processes still contains significant contaminants of arsenic, antimony, bismuth, zinc, copper, silver, and gold. The melt is treated in a reverberatory furnace with air, steam, and sulfur, which oxidizes the contaminants except silver, gold, and bismuth. The oxidized contaminants are removed by drossing, where they float to the top and are skimmed off.[12][14]
Most lead ores contain significant concentrations of silver, resulting in the smelted metal also containing silver as a contaminant. Metallic silver as well as gold is removed and recovered economically by means of the Parkes process.[2][12][14]
Desilvered lead is freed of bismuth according to the Betterton-Kroll process by treating it with metallic calcium and magnesium, which forms a bismuth dross that can be skimmed off.[12][14]
Very pure lead can be obtained by processing smelted lead electrolytically by means of the Betts process. The process uses anodes of impure lead and cathodes of pure lead in an electrolyte of silica fluoride.[12][14]
Production and recycling
Production and consumption of lead is increasing worldwide. Total annual production is about 8 million tonnes; about half is produced from recycled scrap. The top lead producing countries, as of 2008, are Australia, China, USA, Peru, Canada, Mexico, Sweden, Morocco, South Africa and North Korea.[14] Australia, China and the United States account for more than half of primary production.[15]
- 2008 mine production: 3,886,000 tonnes
- 2008 metal production: 8,725,000 tonnes
- 2008 metal consumption: 8,706,000 tonnes[16]
At current use rates, the supply of lead is estimated to run out in 42 years.[17] Environmental analyst, Lester Brown, however, has suggested lead could run out within 18 years based on an extrapolation of 2% growth per year.[18] This may need to be reviewed to take account of renewed interest in recycling, and rapid progress in fuel cell technology.
Applications
Due to its half life of 22.2 years the radioactive isotope 210Pb is used for dating material from marine sediment cores by radiometric methods.
Elemental Lead

Because of its high density and resistance from corrosion, lead is used for the ballast keel of sailboats. Its high density allows it to counterbalance the heeling effect of wind on the sails while at the same time occupying a small volume and thus offering the least underwater resistance. For the same reason it is used in scuba diving weight belts to counteract the diver's natural buoyancy and that of his equipment. It does not have the weight-to-volume ratio of many heavy metals, but its low cost increases its use in these and other applications.



Lead is used in applications where its low melting point, ductility and high density is an advantage. The low melting point makes casting of lead easy, and therefore small arms ammunition and shotgun pellets can be cast with minimal technical equipment. It is also inexpensive and denser than other common metals.[19] The hot metal typesetting uses a lead based alloy to produce the types for printing directly before printing.
Its corrosion resistance makes it suitable for outdoor applications when in contact with water.
More than half of the worldwide lead production is used as electrodes in the lead-acid battery, used extensively as a car battery.
Cathode (reduction)
- PbO2 + 4 H+ + SO42- → PbSO4 + 2 H2O
Anode (oxidation)
Lead is used to form glazing bars for stained glass or other multi-lit windows. The practice has become less common, not for danger but for stylistic reasons. Lead, or sheet-lead, is used as a sound deadening layer in some areas in wall, floor and ceiling design in sound studios where levels of airborne and mechanically produced sound are targeted for reduction or virtual elimination.[22][23]
Lead is used as shielding from radiation (e.g., in X-ray rooms).[24] Molten lead is used as a coolant (e.g., for lead cooled fast reactors).[25]
Lead is the traditional base metal of organ pipes, mixed with varying amounts of tin to control the tone of the pipe.[26][27]
Lead is used as electrodes in the process of electrolysis. Lead is used in solder for electronics, although this usage is being phased out by some countries to reduce the amount of environmentally hazardous waste. Lead is used in high voltage power cables as sheathing material to prevent water diffusion into insulation. Lead is added to brass to reduce machine tool wear. Lead, in the form of strips, or tape, is used for the customization of tennis rackets. Tennis rackets of the past sometimes had lead added to them by the manufacturer to increase weight.[28]
Lead has many uses in the construction industry (e.g., lead sheets are used as architectural metals in roofing material, cladding, flashing, gutters and gutter joints, and on roof parapets). Detailed lead moldings are used as decorative motifs used to fix lead sheet. Lead is still widely used in statues and sculptures. Lead is often used to balance the wheels of a car; this use is being phased out in favor of other materials for environmental reasons.
Lead Compounds
Lead compounds are used as a coloring element in ceramic glazes, notably in the colors red and yellow.[29] Lead is frequently used in polyvinyl chloride (PVC) plastic, which coats electrical cords.[30][31]
Lead is used in some candles to treat the wick to ensure a longer, more even burn. Because of the dangers, European and North American manufacturers use more expensive alternatives such as zinc.[32][33]Lead glass is composed of 12-28% lead oxide. It changes the optical characteristics of the glass and reduces the transmission of radiation.[34]
Some artists using oil-based paints continue to use lead carbonate white, citing its properties in comparison with the alternatives. Tetra-ethyl lead is used as an anti-knock additive for aviation fuel in piston driven aircraft. Lead-based semiconductors, such as lead telluride, lead selenide and lead antimonide are finding applications in photovoltaic (solar energy) cells and infrared detectors.[35]
Former applications
Lead pigments were used in lead paint for white as well as yellow, orange, and red. Most uses have been discontinued due of the dangers of lead poisoning. However, lead chromate is still in industrial use. Lead carbonate (white) is the traditional pigment for the priming medium for oil painting, but it has been largely displaced by the zinc and titanium oxide pigments. It was also quickly replaced in water-based painting mediums. Lead carbonate white was used by the Japanese geisha and in the West for face-whitening make-up, which was detrimental for health.
Lead was the hot metal used in hot metal typesetting. Lead was used for plumbing in Ancient Rome. Lead was used as a preservative for food and drink in Ancient Rome. Lead was used for joining cast iron water pipes and used as a material for small diameter water pipes until the early 1970s.
Tetraethyl lead was used in leaded fuels to reduce engine knocking – however this practice has been phased out across many countries of the world in efforts to reduce toxic pollution that affected humans and the environment.[36][37][38]
Lead was used to make bullets for slings. Lead was used for shotgun pellets in the US until about 1992 when it was outlawed (for waterfowl hunting only) and replaced by non-toxic shot, primarily steel pellets.
Lead as a component of the paint used on children's toys – now restricted in the United States and across Europe (ROHS Directive). Lead was used in car body filler, which was used in many custom cars in the 1940s–60s. Hence the term Leadsled. Lead is a superconductor at 7.2 K and IBM tried to make a Josephson effect computer out of lead-alloy.[39]
Lead was also used in pesticides before the 1950s, when fruit orchards were treated (ATSDR). A lead cylinder attached to a long line was used by sailors for the vital navigational task of determining water depth by heaving the lead at regular internals. A soft tallow insert at its base allowed the nature of the sea bed to be determined, further aiding position finding. Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. The term comes from the Roman stylus, called the penicillus, which was made of lead without a wooden holder.[40] When the pencil originated as a wrapped graphite writing tool, the particular type of graphite being used was named plumbago (lit. act for lead, or lead mockup).
Mapping of industrial releases in the United States
One tool that maps releases of lead [1] to particular locations in the United States[41] and also provides additional information about such releases is TOXMAP. TOXMAP is a Geographic Information System (GIS) from the Division of Specialized Information Services of the United States National Library of Medicine (NLM) that uses maps of the United States to help users visually explore data from the United States Environmental Protection Agency's (EPA) Toxics Release Inventory and Superfund Basic Research Programs. TOXMAP is a resource funded by the US Federal Government. TOXMAP's chemical and environmental health information is taken from NLM's Toxicology Data Network (TOXNET)[42] and PubMed, and from other authoritative sources.
Health effects
Lead is a poisonous metal that can damage nervous connections (especially in young children) and cause blood and brain disorders. Lead poisoning typically results from ingestion of food or water contaminated with lead; but may also occur after accidental ingestion of contaminated soil, dust, or lead based paint.[43] Long-term exposure to lead or its salts (especially soluble salts or the strong oxidant PbO2) can cause nephropathy, and colic-like abdominal pains. The effects of lead are the same whether it enters the body through breathing or swallowing. Lead can affect almost every organ and system in the body. The main target for lead toxicity is the nervous system, both in adults and children. Long-term exposure of adults can result in decreased performance in some tests that measure functions of the nervous system. It may also cause weakness in fingers, wrists, or ankles. Lead exposure also causes small increases in blood pressure, particularly in middle-aged and older people and can cause anemia. Exposure to high lead levels can severely damage the brain and kidneys in adults or children and ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure have shown to reduce fertility in males.[44] The antidote/treatment for lead poisoning consists of dimercaprol and succimer.
The concern about lead's role in cognitive deficits in children has brought about widespread reduction in its use (lead exposure has been linked to learning disabilities).[45] Most cases of adult elevated blood lead levels are workplace-related.[46] High blood levels are associated with delayed puberty in girls.[47] Lead has been shown many times to permanently reduce the cognitive capacity of children at extremely low levels of exposure.[48] There appears to be no detectable lower limit below which lead has no effect on cognition.
During the 20th century, the use of lead in paint pigments was sharply reduced because of the danger of lead poisoning, especially to children.[49][50] By the mid-1980s, a significant shift in lead end-use patterns had taken place. Much of this shift was a result of the U.S. lead consumers' compliance with environmental regulations that significantly reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems. Lead use is being further curtailed by the European Union's RoHS directive. Lead may still be found in harmful quantities in stoneware, vinyl (such as that used for tubing and the insulation of electrical cords), and brass manufactured in China. Between 2006 and 2007 many children's toys made in China were recalled, primarily due to lead in paint used to color the product.
Older houses may still contain substantial amounts of lead paint. White lead paint has been withdrawn from sale in industrialized countries, but the yellow lead chromate is still in use; for example, Holland Colours Holcolan Yellow. Old paint should not be stripped by sanding, as this produces inhalable dust.
Lead salts used in pottery glazes have on occasion caused poisoning, when acidic drinks, such as fruit juices, have leached lead ions out of the glaze.[51] It has been suggested that what was known as "Devon colic" arose from the use of lead-lined presses to extract apple juice in the manufacture of cider. Lead is considered to be particularly harmful for women's ability to reproduce. Lead(II) acetate (also known as sugar of lead) was used by the Roman Empire as a sweetener for wine, and some consider this to be the cause of the dementia that affected many of the Roman Emperors.[52]
Lead as a soil contaminant is a widespread issue, since lead is present in natural deposits and may also enter soil through (leaded) gasoline leaks from underground storage tanks or through a wastestream of lead paint or lead grindings from certain industrial operations.
Lead can also be found listed as a criteria pollutant in the United States Clean Air Act section 108. Lead that is emitted into the atmosphere can be inhaled, or it can be ingested after it settles out of the air. It is rapidly absorbed into the bloodstream and is believed to have adverse effects on the central nervous system, the cardiovascular system, kidneys, and the immune system.[53]
Biochemistry of lead poisoning
In the human body, lead inhibits porphobilinogen synthase and ferrochelatase, preventing both porphobilinogen formation and the incorporation of iron into protoporphyrin IX, the final step in heme synthesis. This causes ineffective heme synthesis and subsequent microcytic anemia.[54] At lower levels, it acts as a calcium analog, interfering with ion channels during nerve conduction. This is one of the mechanisms by which it interferes with cognition. Acute lead poisoning is treated using disodium calcium edetate: the calcium chelate of the disodium salt of ethylene-diamine-tetracetic acid (EDTA). This chelating agent has a greater affinity for lead than for calcium and so the lead chelate is formed by exchange. This is then excreted in the urine leaving behind harmless calcium.[55]
Leaching of lead from metal surfaces
It is clear from the Pourbaix diagram below that lead is more likely to corrode in a citrate medium than it is in a non-complexing medium. The central part of the diagram shows that lead metal oxidizes more easily in the citrate medium than in normal water.
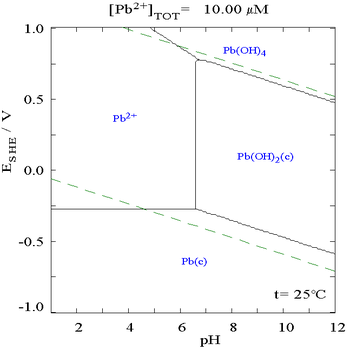 |
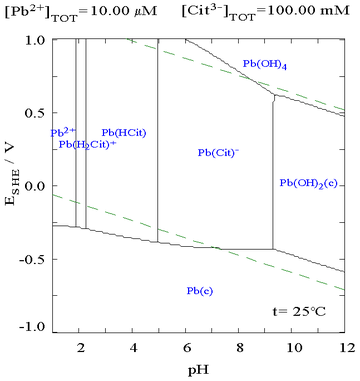 |
| The Pourbaix diagram for lead in a non-complexing aqueous medium (e.g., perchloric acid/sodium hydroxide)[5] | The Pourbaix diagram for lead in citric acid/citrate[5] |
In a Pourbaix diagram, the acidity is plotted on the x axis using the pH scale, while how oxidizing/reducing nature of the system is plotted on the y axis in terms of volts relative to the standard hydrogen electrode. The diagram shows the form of the element which is most chemically stable at each point, it only comments on thermodynamics and it says nothing about the rate of change (kinetics).
Exposure pathways
Exposure to lead and lead chemicals can occur through inhalation, ingestion and dermal contact. Most exposure occurs through ingestion or inhalation; in the U.S. the skin exposure is unlikely as leaded gasoline additives are no longer used. Lead exposure is a global issue as lead mining and lead smelting are common in many countries. Most countries have stopped using lead-containing gasoline by 2007.[56]
Lead exposure mostly occurs through ingestion. Lead paint is the major source of lead exposure for children. As lead paint deteriorates, it peels, pulverizes and then enters the body through hand-to-mouth contacts or through contaminated food, water or alcohol. Ingesting certain home remedy medicines may also expose people to lead or lead compounds.[56] Lead can be ingested through fruits and vegetables contaminated by high levels of lead in the soils. Soil is contaminated due to the lead in pipes, lead dust from old paints and residual lead from gasoline with lead that was used before the Environment Protection Agency issue the regulation around 1980.[57]
Inhalation is the second major pathway of exposure, especially for workers in lead-related occupations. Almost all inhaled lead is absorbed into the body, the rate is 20-70% for ingested lead; children absorb more than adults.[56]
Dermal exposure may be significant for a narrow category of people working with organic lead compounds, but is of little concern for general population. The rate of skin absorption is also low for inorganic lead.[56]
Lead remains in the body for long periods in mineralizing tissue (i.e., teeth and bones). The stored lead may be released into the bloodstream, especially in times of calcium stress (e.g., pregnancy, lactation, osteoporosis), or calcium deficiency, and is of particular risk to the developing fetus.[56]
Occupational exposure
Lead is widely used in the production of batteries, metal products (solder and pipes), ammunition and devices to shield X-rays leading to its exposure to the people working in these industries. Use of lead in gasoline, paints and ceramic products, caulking, and pipe solder has been dramatically reduced in recent years because of health concerns. Ingestion of contaminated food and drinking water is the most common source of lead exposure in humans. Exposure can also occur via inadvertent ingestion of contaminated soil/dust or lead-based paint.
Testing
Water contamination can be tested with commercially available kits. Analysis of lead in whole blood is the most common and accurate method of assessing lead exposure in human. Erythrocyte protoporphyrin (EP) tests can also be used to measure lead exposure, but are not as sensitive at low blood lead levels (<0.2 mg/L). Lead in blood reflects recent exposure. Bone lead measurements are an indicator of cumulative exposure. While measurements of urinary lead levels and hair have been used to assess lead exposure, they are not reliable.
See also
- Adult Blood Lead Epidemiology and Surveillance
- Lead-Free Toys Act
- Medical geology
- Plumbosolvency
- RoHS directive
- Banning of leaded petrol
References
- ↑ Georges, Audi (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ↑ 2.0 2.1 2.2 2.3 Linus, Pauling (1947). General Chemistry. W.H. Freeman.
- ↑ 3.0 3.1 Brady, James E. and Holum, John R. (1996). Descriptive Chemistry of the Elements. John Wiley and Sons. ISBN 0471135577.
- ↑ Windholz, Martha (1976). Merck Index of Chemicals and Drugs, 9th ed., monograph 8393. Merck. ISBN 0911910263.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Puigdomenech, Ignasi (2004). Hydra/Medusa Chemical Equilibrium Database and Plotting Software. KTH Royal Institute of Technology. http://www.kemi.kth.se/medusa/.
- ↑ Ward, C.H.; Hlousek, Douglas A.; Phillips, Thomas A.; Lowe, Donald F. (2000). Remediation of Firing Range Impact Berms. CRC Press. ISBN 1566704626.
- ↑ Heskel, Dennis L. (1983). "A Model for the Adoption of Metallurgy in the Ancient Middle East". Current Anthropology 24 (3): 362–366. doi:10.1086/203007.
- ↑ 8.0 8.1 Hong, Sungmin; Candelone, Jean-Pierre; Patterson, Clair C.; Boutron, Claude F. (1994): "Greenland Ice Evidence of Hemispheric Lead Pollution Two Millennia Ago by Greek and Roman Civilizations", Science, Vol. 265, No. 5180, pp. 1841–1843
- ↑ Callataÿ, François de (2005): "The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks", Journal of Roman Archaeology, Vol. 18, pp. 361–372 (361–365)
- ↑ Settle, Dorothy M.; Patterson, Clair C. (1980): "Lead in Albacore: Guide to Lead Pollution in Americans", Science, Vol. 207, No. 4436, pp. 1167–1176 (1170f.)
- ↑ Squatriti, Paolo, ed (2000). Working with water in medieval Europe : technology and resource use. Leiden: Brill. pp. 134f.. ISBN 9789004106802.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 Samans, Carl H. (1949). Engineering Metals and their Alloys. MacMillan.
- ↑ "Primary Extraction of Lead Technical Notes". LDA International. http://www.ldaint.org/technotes1.htm. Retrieved 7 April 2007.
- ↑ 14.0 14.1 14.2 14.3 14.4 "Primary Lead Refining Technical Notes". LDA International. http://www.ldaint.org/technotes2.htm. Retrieved 7 April 2007.
- ↑ "Lead Information". LDA International. http://www.ldaint.org/information.htm. Retrieved 2007-09-05.
- ↑ "Lead and Zinc Statistics". International Lead and Zinc Study Group. http://www.ilzsg.org/static/statistics.aspx?from=1. Retrieved 2009-02-19.
- ↑ "How Long Will it Last?". New Scientist 194 (2605): 38–39. May 26, 2007. ISSN 0262-4079.
- ↑ Brown, Lester (2006). Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble. New York: W.W. Norton. p. 109. ISBN 0393328317.
- ↑ Rooney, Corinne. "Contamination at Shooting Ranges" (PDF). The Lead Group, incorporated. http://www.lead.org.au/fs/shootingranges.pdf. Retrieved 7 April 2007.
- ↑ T.R. Crompton. (2000). Battery reference book. Oxford, England: Newnes. pp. 18/2–18/4. ISBN 9780750646253. http://books.google.com/?id=q58IX4BM7-0C&pg=PT210.
- ↑ Stellman, Jeanne Mager (1998). Encyclopaedia of Occupational Health and Safety. International Labour Organization. pp. 81.2–81.4. ISBN 9789221098164. http://books.google.com/?id=nDhpLa1rl44C&pg=PT644.
- ↑ Guruswamy, Sivaraman (2000). Engineering properties and applications of lead alloys. New York, NY: Marcel Dekker. p. 31. ISBN 9780824782474. http://books.google.com/?id=gDwOAAAAQAAJ&pg=PA31.
- ↑ Lansdown, Richard and Yule, William, ed (1986). The Lead debate : the environment, toxicology, and child health. London: Croom Helm. p. 240. ISBN 9780709916536. http://books.google.com/?id=TtGmjOv9CUAC&pg=PA240.
- ↑ Structural shielding design for medical X-ray imaging facilities.. Bethesda, MD: National Council on Radiation Protection and Measurement. 2004. pp. 16–17. ISBN 9780929600833. http://books.google.com/?id=DKu4YDjEluoC&pg=PA16.
- ↑ Tuček, Kamil; Carlsson, Johan; Wider, Hartmut (2006). "Comparison of sodium and lead-cooled fast reactors regarding reactor physics aspects, severe safety and economical issues". Nuclear Engineering and Design 236: 1589. doi:10.1016/j.nucengdes.2006.04.019. http://www.ecolo.org/documents/documents_in_english/SFRvsLFR-05.pdf.
- ↑ Audsley, George Ashdown (1988-04-01). The Art of Organ Building, Vol. 2. pp. 250–251. ISBN 9780486213156. http://books.google.com/?id=I0h525OVoTgC&pg=PA503.
- ↑ Palmieri, Robert, ed (2006). The Organ. New York u.a.: Garland. pp. 412–413. ISBN 9780415941747. http://books.google.com/?id=cgDJaeFFUPoC&pg=PA412.
- ↑ Hong, Youlian and Bartlett, Roger, ed (2008). Routledge Handbook of Biomechanics and Human Movement Science. London: Routledge. p. 250. ISBN 9780415408813. http://books.google.com/?id=p7Ne5IjK5H0C&pg=PA250.
- ↑ Leonard, Alvin R.; Lynch, Glenn (1958). "Dishware as a Possible Source for Lead Poisoning". Calif. Med. 89 (6): 414–416. PMID 13608300.
- ↑ Zweifel, Hans (2009). Plastics Additives Handbook. Hanser Verlag. pp. 438. ISBN 9783446408012. http://books.google.com/?id=WbBH5QFXOhoC&pg=PT475.
- ↑ Wilkes, C. E.; Summers, J. W.; Daniels, C. A.; Berard, M. T. (2005). PVC handbook. München: Hanser. p. 106. ISBN 9781569903797. http://books.google.com/?id=YUkJNI9QYsUC&pg=PA106.
- ↑ Randerson, James (June 2002). "Candle pollution". NewScientist.com (2348). http://www.newscientist.com/article/mg17423481.900-candle-pollution.html. Retrieved 2007-04-07.
- ↑ Nriagu, J; Kim, MJ (2000). "Emissions of lead and zinc from candles with metal-core wicks". The Science of the Total Environment 250 (1-3): 37. doi:10.1016/S0048-9697(00)00359-4. PMID 10811249.
- ↑ Amstock, Joseph S. (1997). Handbook of glass in construction. McGraw-Hill Professional. pp. 116–119. ISBN 9780070016194. http://books.google.com/?id=apWvKnKKrvsC&pg=PA116.
- ↑ "Applications for Lead". http://www.americanelements.com/pb.html. Retrieved 7 April 2007.
- ↑ "Lead replacement petrol phase-out – Information to motorists". Department for Transport (gov.uk). http://www.dft.gov.uk/pgr/roads/environment/lrp/leadreplacementpetrolphaseout.
- ↑ "National phase out of leaded petrol: Some questions and answers". Department of the Environment and Heritage, Australian Government. 2001. http://www.environment.gov.au/atmosphere/airquality/publications/qa.html.
- ↑ "Banning of Leaded Gasoline for Highway Use". Knight Ridder/Tribune Business News. 4 October 1995. http://www.accessmylibrary.com/coms2/summary_0286-6346110_ITM. Retrieved 23 September 2008.
- ↑ Henkels, W. H.; Geppert, L. M.; Kadlec, J.; Epperlein, P. W.; Beha, H. (September 1985). "Josephson 4 K-bit cache memory design for a prototype signal processor.". Harvard University. http://adsabs.harvard.edu/abs/1985JAP....58.2371H. Retrieved 7 April 2007.
- ↑ "A history of pencils". www.pencils.com. http://www.pencils.com/history.html. Retrieved 7 April 2007.
- ↑ "TRI Releases Map". Toxmap.nlm.nih.gov. http://toxmap.nlm.nih.gov/. Retrieved 2010-03-23.
- ↑ TOXNET – Databases on toxicology, hazardous chemicals, environmental health, and toxic releases
- ↑ "ToxFAQs: CABS/Chemical Agent Briefing Sheet: Lead.". Agency for Toxic Substances and Disease Registry/Division of Toxicology and Environmental Medicine. 2006. http://www.atsdr.cdc.gov/cabs/lead/lead_cabs.pdf.
- ↑ Golub, Mari S., ed (2005). "Summary". Metals, fertility, and reproductive toxicity. Boca Raton, Fla.: Taylor and Francis. p. 153. ISBN 9780415700405. http://books.google.com/?id=Qt8LEB7_HyQC&pg=PA153.
- ↑ Hu, Howard (1991). "Knowledge of diagnosis and reproductive history among survivors of childhood plumbism". American Journal of Public Health 81 (8): 1070–1072. doi:10.2105/AJPH.81.8.1070. PMID 1854006.
- ↑ "NIOSH Adult Blood Lead Epidemiology and Surveillance". United States National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/topics/ABLES/ables-description.html. Retrieved 2007-10-04.
- ↑ Schoeters, G.; et al. (2008). "Endocrine Disruptors and Abnormalities of Pubertal Development". Basic & Clinical Pharmacology & Toxicology 102 (2): 168–175. doi:10.1111/j.1742-7843.2007.00180.x (inactive 2009-12-12). PMID 18226071. http://www3.interscience.wiley.com/journal/119425382/abstract.
- ↑ Needleman, H.L. ; Schell, A.; Bellinger, D.; Leviton, A.; Allred, E. N. (1990). "The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report". New England Journal of Medicine 322 (2): 83–88. PMID 2294437. http://content.nejm.org/cgi/content/abstract/322/2/83.
- ↑ "Download: Lead paint: Cautionary note". Queensland Government. http://www.derm.qld.gov.au/heritage/owning_a_heritage_place/general_exemptions/g1__maintenance_and_minor_repair/lead_paint_cautionary_note.html. Retrieved 7 April 2007.
- ↑ "Lead Paint Information". Master Painters, Australia. http://www.qld.mpa.org.au/index.php/content/33/. Retrieved 7 April 2007.
- ↑ "CPG Sec. 545.450 Pottery (Ceramics); Import and Domestic – Lead Contamination". U.S. Food and Drug Administration. http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm074516.htm. Retrieved 2010-02-02.
- ↑ Angier, Natalie (August 21, 2007). "The Pernicious Allure of Lead". New York Times. http://www.nytimes.com/2007/08/21/science/21angi.html?_r=1&oref=slogin. Retrieved 7 May 2010.
- ↑ Bergeson, Lynn L. (2008). "The proposed lead NAAQS: Is consideration of cost in the clean air act's future?". Environmental Quality Management 18: 79. doi:10.1002/tqem.20197.
- ↑ Cohen, Alan R.; Trotzky, Margret S.; Pincus, Diane (1981). "Reassessment of the Microcytic Anemia of Lead Poisoning". Pediatrics 67 (6): 904–906. PMID 7232054. http://pediatrics.aappublications.org/cgi/content/abstract/67/6/904.
- ↑ Laurence, D.R. (1966). Clinical Pharmacology(Third Edition).
- ↑ 56.0 56.1 56.2 56.3 56.4 Case Studies in Environmental Medicine Lead (Pb) Toxicity: How are People Exposed to Lead?
- ↑ "Information for the Community Lead Toxicity". Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/csem/lead/community/index.html.
Further reading
- Keisch, B., Feller, R. L., Levine, A. S., and Edwards, R. R.: "Dating and Authenticating Works of Art by Measurement of Natural Alpha Emitters". Science, 155, No. 3767, p. 1238–1242, 1967.
- Keisch, B: "Dating Works of Art Through their Natural Radioactivity: Improvements and Applications". Science, 160, p. 413–415, 1968.
- Keisch, B: "Discriminating Radioactivity Measurements of Lead: New Tool for Authentication". Curator, 11, No. 1., p. 41–52, 1968.
- Jose S. Casas and Jose Sordo. Lead Chemistry, Analytical Aspects. Environmental Impacts and Health Effects, 2006, Elsevier ISBN 0-444-52945-4
External links
- ATSDR Case Studies in Environmental Medicine: Lead Toxicity U.S. Department of Health and Human Services
- Lead at the Open Directory Project
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Lead
- Lead-Free Wheels
- National Lead Free Wheel Weight Initiative| Waste Minimization|Wastes|US EPA
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
